Exhibit 99.1
Adaptimmune Transforming T-cell Therapy Presentation Materials December 2015
Exhibit 99.1
Adaptimmune Transforming T-cell Therapy Presentation Materials December 2015

Disclaimer This presentation contains “forward-looking statements,” as that term is defined under the Private Securities Litigation Reform Act of 1995 (PSLRA), which statements may be identified by words such as “believe,” “may”, “will,” “estimate,” “continue,” “anticipate,” “intend,” “expect” and other words of similar meaning. These forward-looking statements involve certain risks and uncertainties. Such risks and uncertainties could cause our actual results to differ materially from those indicated by such forward-looking statements, and include, without limitation: the success, cost and timing of our product development activities and clinical trials; our ability to submit an IND and successfully advance our technology platform to improve the safety and effectiveness of our existing TCR therapeutic candidates; the rate and degree of market acceptance of T-cell therapy generally and of our TCR therapeutic candidates; government regulation and approval, including, but not limited to, the expected regulatory approval timelines for TCR therapeutic candidates; and our ability to protect our proprietary technology and enforce our intellectual property rights; amongst others. For a further description of the risks and uncertainties that could cause our actual results to differ materially from those expressed in these forward-looking statements, as well as risks relating to our business in general, we refer you to our Annual Report on Form 20-F filed with the Securities and Exchange Commission (SEC) on October 13, 2015 and our other SEC filings. We urge you to consider these factors carefully in evaluating the forward-looking statements herein and are cautioned not to place undue reliance on such forward-looking statements, which are qualified in their entirety by this cautionary statement. The forward-looking statements contained in this presentation speak only as of the date the statements were made and we do not undertake any obligation to update such forward-looking statements to reflect subsequent events or circumstances. We intend that all forward-looking statements be subject to the safe-harbor provisions of the PSLRA.
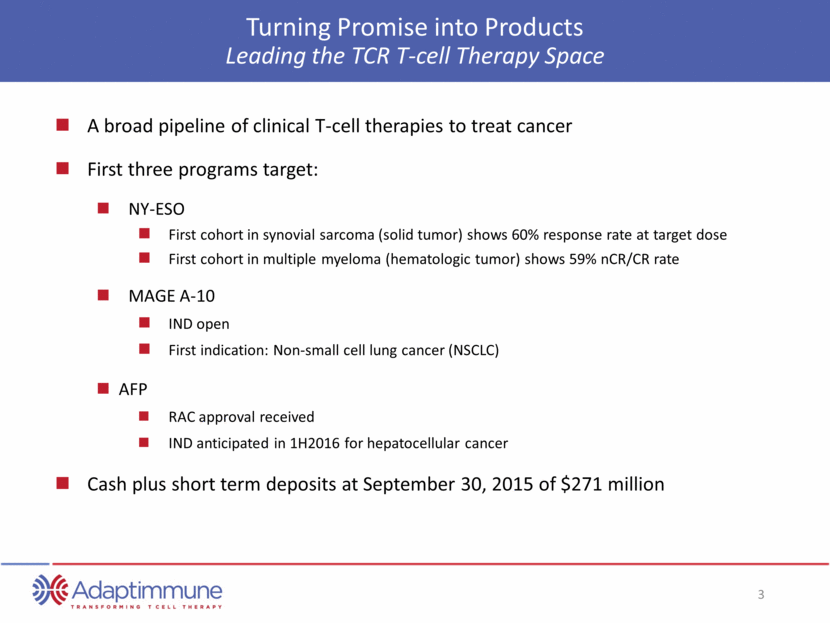
Turning Promise into Products Leading the TCR T-cell Therapy Space A broad pipeline of clinical T-cell therapies to treat cancer First three programs target: NY-ESO First cohort in synovial sarcoma (solid tumor) shows 60% response rate at target dose First cohort in multiple myeloma (hematologic tumor) shows 59% nCR/CR rate MAGE A-10 IND open First indication: Non-small cell lung cancer (NSCLC) AFP RAC approval received IND anticipated in 1H2016 for hepatocellular cancer Cash plus short term deposits at September 30, 2015 of $271 million
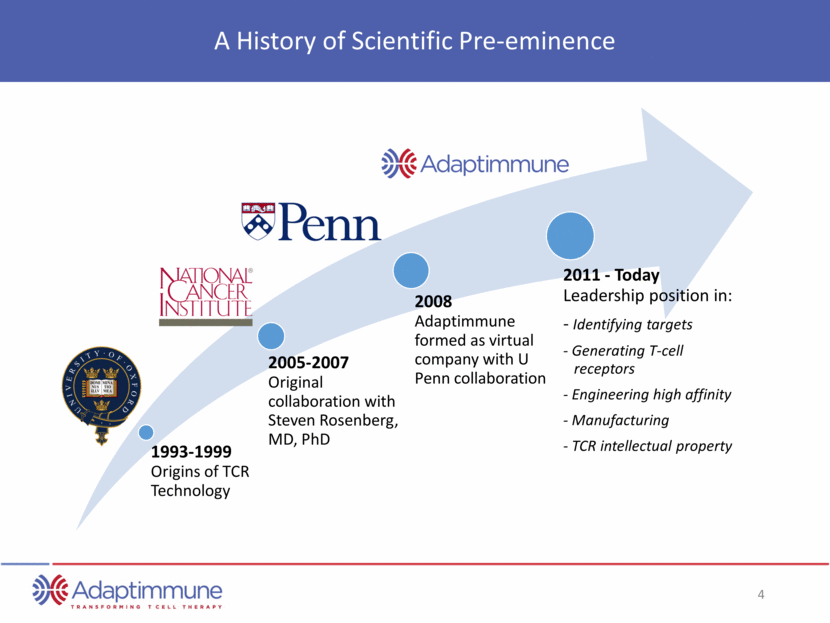
A History of Scientific Pre-eminence 1993-1999 Origins of TCR Technology 2005-2007 Original collaboration with Steven Rosenberg, MD, PhD 2008 Adaptimmune formed as virtual company with U Penn collaboration 2011 - Today Leadership position in: - Identifying targets - Generating T-cell receptors - Engineering high affinity - Manufacturing - TCR intellectual property
Two Years of Delivery $104 million Series A Financing Cancer Immunotherapy collaboration Successful IPO raises $176 million Presentation of clinical data from multiple studies of NY-ESO therapy FDA acceptance of MAGE-A10 IND Updated data on NY-ESO therapy in synovial sarcoma and multiple myeloma Universal Cells collaboration on allogeneic T-cell therapies May 2014 35 people <$2 million September 2015 ~200 people $271 million Today 2014 2015
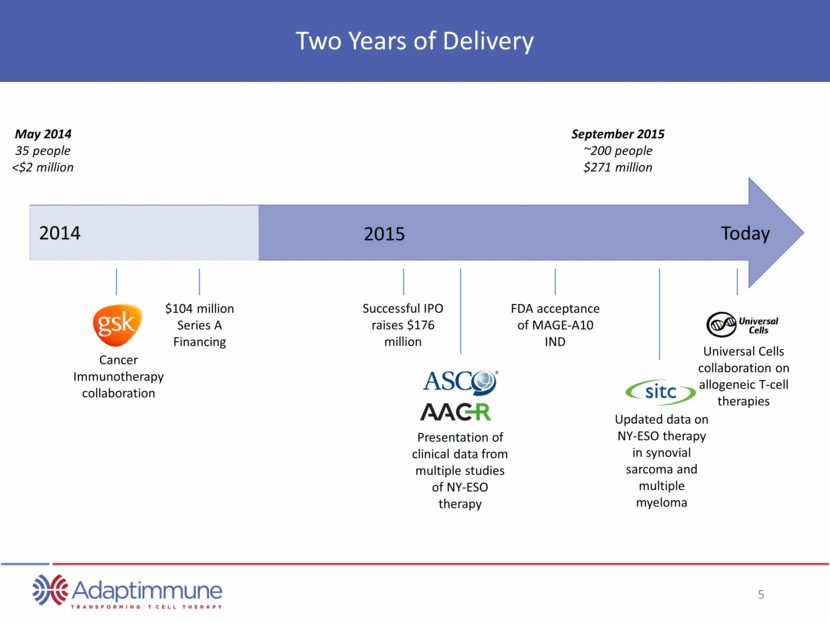
Transformative Six Months Since IPO Rapidly Executing on the Promise of Immuno-oncology July: Publication of clinical and persistence data of NY-ESO in multiple myeloma September: Expansion of study of NY-ESO therapy in synovial sarcoma July: FDA acceptance of MAGE-A10 IND November: Updated data on NY-ESO therapy in synovial sarcoma and multiple myeloma December: Universal Cells collaboration on allogeneic T-cell therapies October: Groundbreaking of new research (UK) and manufacturing facilities (US) November: AFP protocol approved by NIH’s Recombinant DNA Advisory Committee (RAC) November: Initiation of NSCLC study with NY-ESO TCR December July 2015 December: Initiation of NSCLC study with MAGE A10 TCR expected
Positioned for Success Experienced Management Team James Noble, MA, FCA Chief Executive Officer Helen Tayton-Martin, PhD MBA Chief Operating Officer Adrian Rawcliffe Chief Financial Officer Rafael Amado, MD Chief Medical Officer Gwen Binder-Scholl, PhD EVP, Adaptimmune LLC Head of Translational Sciences

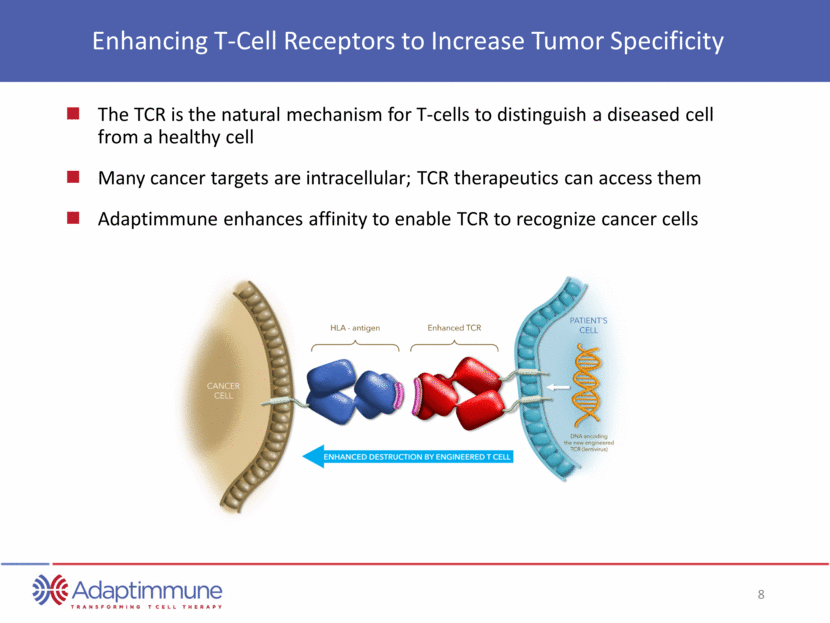
Enhancing T-Cell Receptors to Increase Tumor Specificity The TCR is the natural mechanism for T-cells to distinguish a diseased cell from a healthy cell Many cancer targets are intracellular; TCR therapeutics can access them Adaptimmune enhances affinity to enable TCR to recognize cancer cells
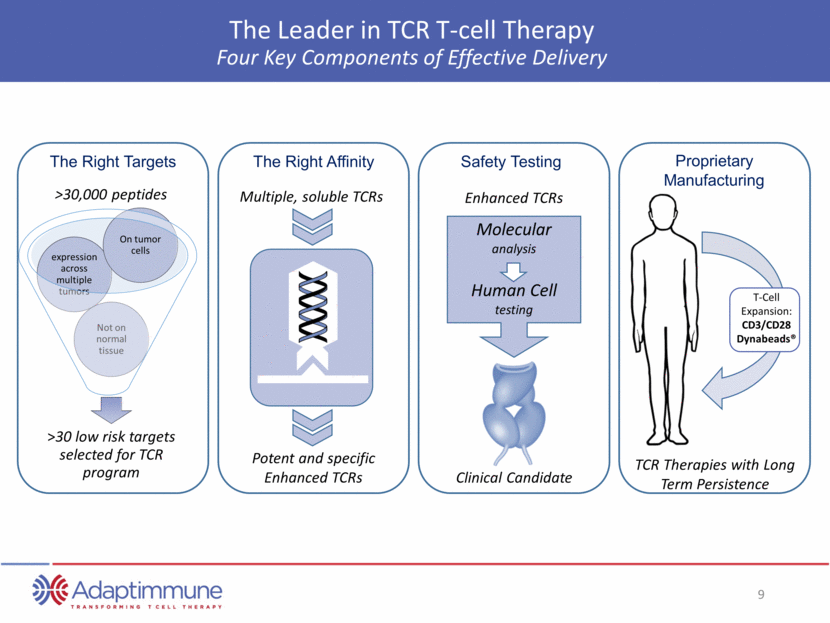
The Leader in TCR T-cell Therapy Four Key Components of Effective Delivery The Right Affinity Safety Testing Proprietary Manufacturing The Right Targets T-Cell Expansion: CD3/CD28 Dynabeads® Multiple, soluble TCRs Potent and specific Enhanced TCRs Enhanced TCRs Clinical Candidate >30,000 peptides Molecular analysis Human Cell testing TCR Therapies with Long Term Persistence >30 low risk targets selected for TCR program Not on normal tissue expression across multiple tumors On tumor cells
Uniquely Positioned for Success Solving Development Hurdles Low affinity of natural T-cells Safety evaluation of T-cell therapies Generating T-cell receptors (TCRs) Long term persistence and response Inability to target solid tumors Proprietary affinity optimization technology Proprietary preclinical safety testing platform Proprietary method of making TCRs for any target Persistence of affinity enhanced T-cells out to three years seen in early data Encouraging clinical activity in solid tumor cells Access to targets on most tumors TCRs can access targets on most solid and hematologic cancers Hurdles The Adaptimmune Solution Efficient expansion of engineered cells Exclusive TCR license to CD3/CD28 Dynabeads®
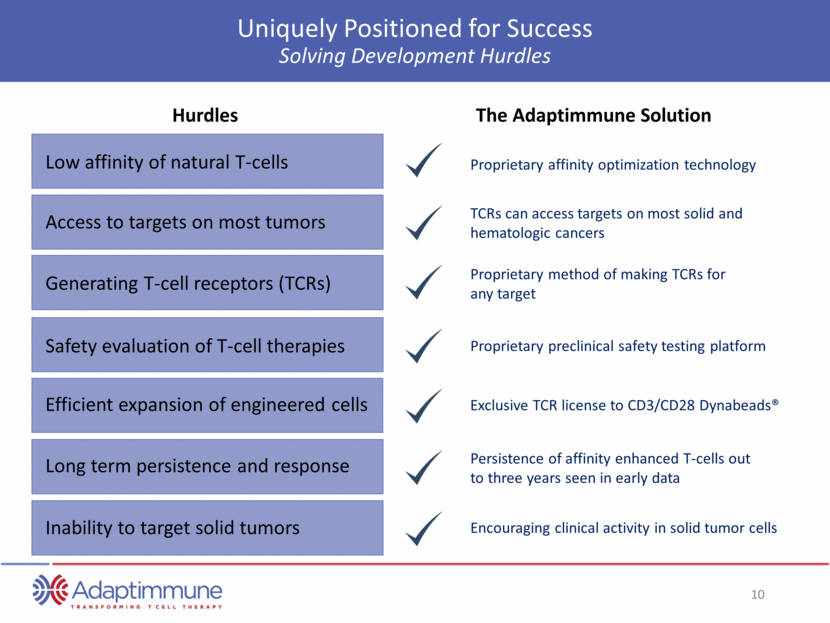
Uniquely Positioned for Success Systematic Approach for Future Improvements Other enhancements to activity Allogeneic T-cell therapies Second generation T-cell therapies Collaboration with Universal Cells Combination studies Beginning in 2016 Opportunities The Adaptimmune Solution Second generation T-cell therapies Impact of tumor microenvironment Enhancements to manufacturing Streamlining process; planning for automation Manufacturing capacity Pilot plant under construction
Targets, Programs and Data

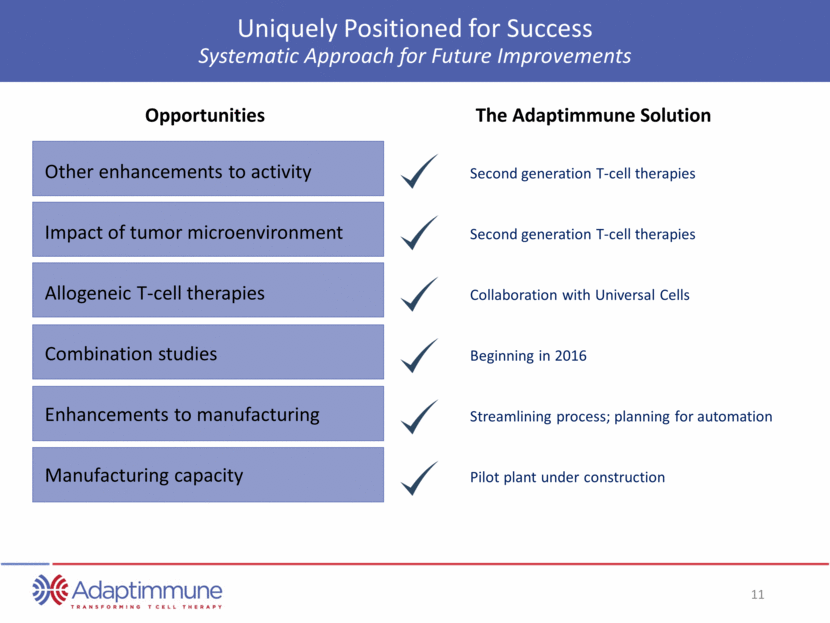
TCR Targets Cover Broad Array of Solid and Hematologic Tumors Sarcoma Melanoma Myeloma Lung Ovarian Esophagogastric Breast Others NY-ESO-1 MAGE A-10 Head and neck Bladder Lung Breast Ovarian Melanoma Cervical Uterine Others AFP AFP Hepatocellular carcinoma / hepatoma AFP 12 other targets in research Multiple targets on most solid and hematologic cancers
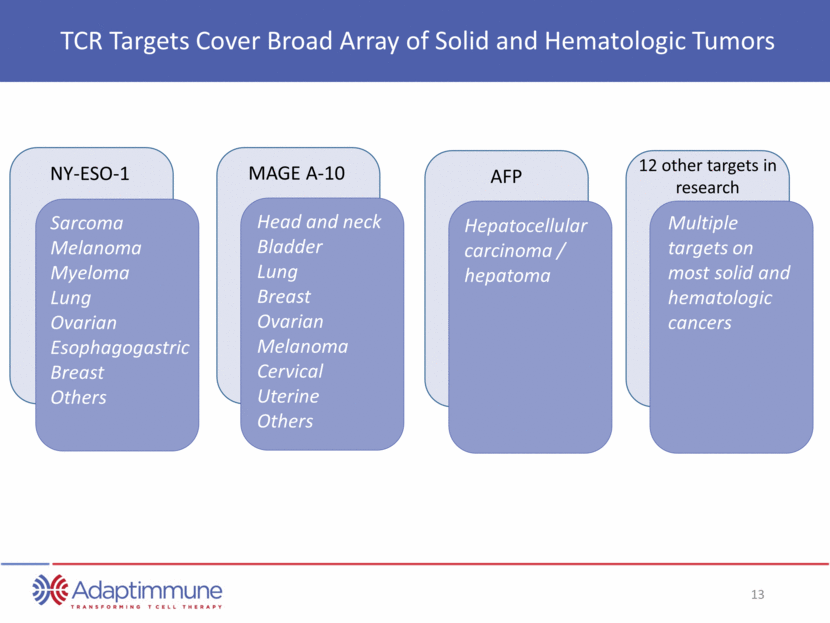
Indication Research Pre-IND Phase 1/2 GSK option to license Industry Leading TCR Pipeline in Solid and Hematologic Cancers Comprehensive Development Program for NY-ESO T-cell Therapy Cohort 1: High NY-ESO expression, 12 patients Esophageal Synovial Sarcoma Cohort 2: Low NY-ESO expression, 10 patients Cohort 3: Removal of fludarabine, 10 patients Cohort 1: Autologous SCT, 25 patients. Data published in N.Med. Multiple myeloma Cohort 2: No autologous SCT, 10 patients 10 pts; 1800 mg/m2/day x 2 days Cy conditioning Ovarian Synovial Sarcoma 6 patients Melanoma Paused pending investigation of day 46 death Esophageal Non-small cell lung cancer 10 pts, Stage IIIb / IV NSCLC; enrollment in 2H15 Investigator initiated study Status Complete Enrolling Enrolling Complete Ongoing Enrolling Enrolling Initiated Q4 2015 Voluntarily Paused
MAGE-A10 TCR Indication Research Pre-IND Phase 1/2 Industry Leading TCR Pipeline in Solid and Hematologic Cancers Deepest Pipeline of Wholly-owned Targets IND open Non-small cell lung cancer (NSCLC) Basket study: Solid tumors Safety testing ongoing; IND planned 1H16 Hepatocellular cancer Synovial Sarcoma 12 undisclosed cancer targets AFP TCR Research programs Adaptimmune 30 undisclosed cancer targets Validated targets Status Initiation Q4 2015 Enrollment in 2016 IND 2017 IND planned 1H 2106 INDs from 2017+ Generation 2 Research & Safety testing ongoing
World Class Clinical Sites

Clinical Data Summary

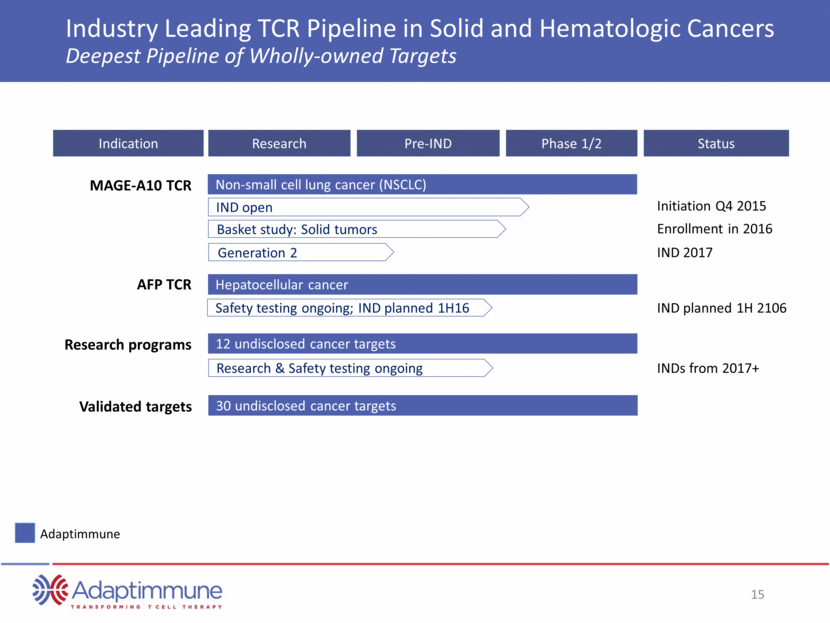
ADAP Phase I/II Study in Synovial Sarcoma Encouraging Response Rates, Tolerability and Persistence 60% response rate in the 10 patients who received target cell dose (at least 1x109 NY-ESO-1C259T cells) 50% overall response rate (6/12) in patients receiving any dose of cells 75% (9/12) of all patients - and 90% (9/10) patients who received target dose - are alive and on long term follow-up. Excludes 3 subjects who did not receive a T-Cell Infusion. One subject with disease progression is excluded because lesion could not be measured *Subjects did not receive the target cell dose SITC November 2015 60 50 40 30 20 10 0 - 10 - 20 - 30 - 40 - 50 - 60 - 70 - 80 - 90 - 100 - 110 - 120 261* 230 206* 207 200 204 202 209 205 208 201 Subject Number Best Response STABLE DISEASE CONFIRMED COMPLETE OR PARTIAL RESPONSE 17 15 - 15 - 16 - 26 - 50 - 55 - 58 - 64 - 70 - 100 C h a nge in T ar ge t Le s ion From Base lin e (%)
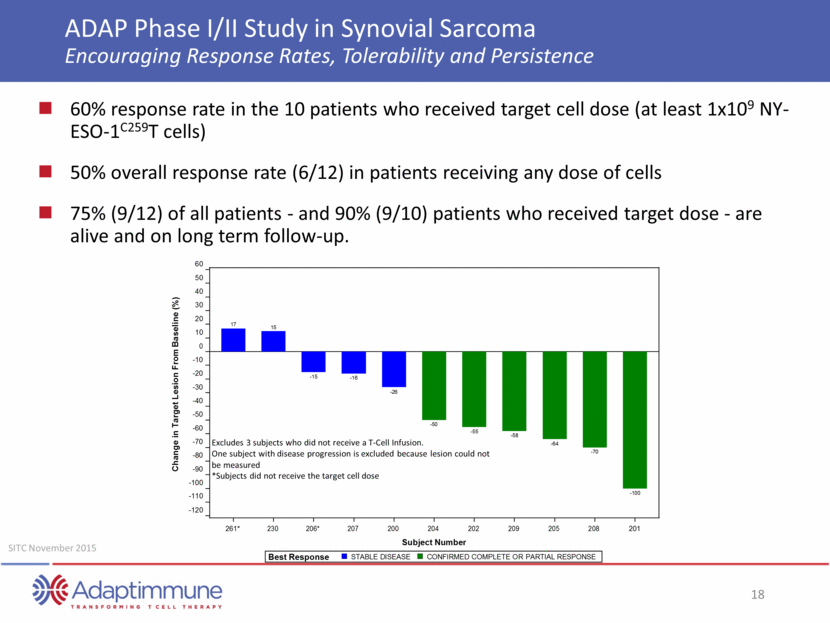
ADAP Phase I/II Study in Synovial Sarcoma Radiographic Pseudoprogression and Response of Lung Metastases Baseline: Bilateral Miliary Metastatic disease Day +2: Pseudoprogression due to Immune Infilration Day +101: Complete Response Patient 201 NY-ESO C-Reactive Protein AACR April 2015
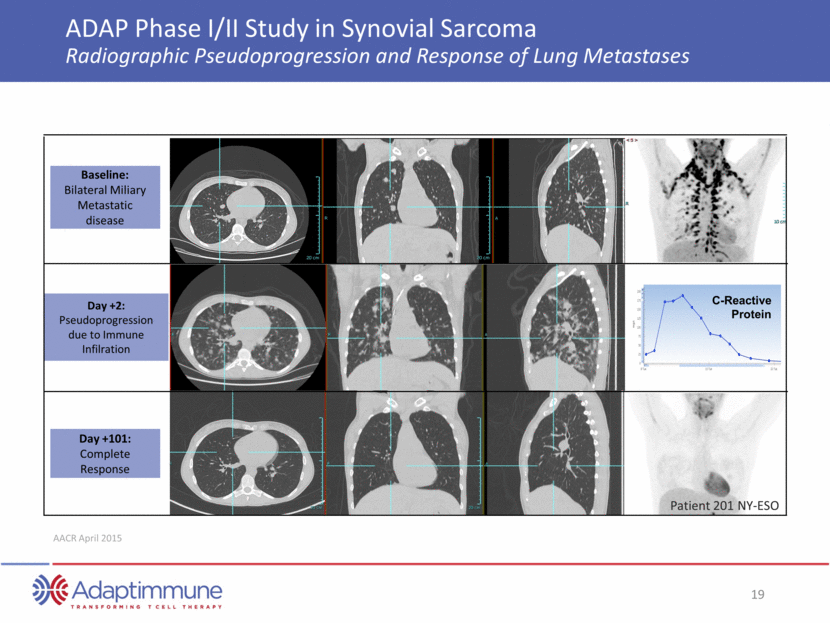
NY-ESO TCR T cells administered ADAP Phase I/II Study in Synovial Sarcoma The Power of T-cell Therapy One month post NY-ESO TCR T cells Two months post NY-ESO T cells Source: Merchant, CTOS, 2014 ~ 70% reduction in lesion size at 2 months after administration of NY-ESO T-cells The lesion was then deemed capable of resection
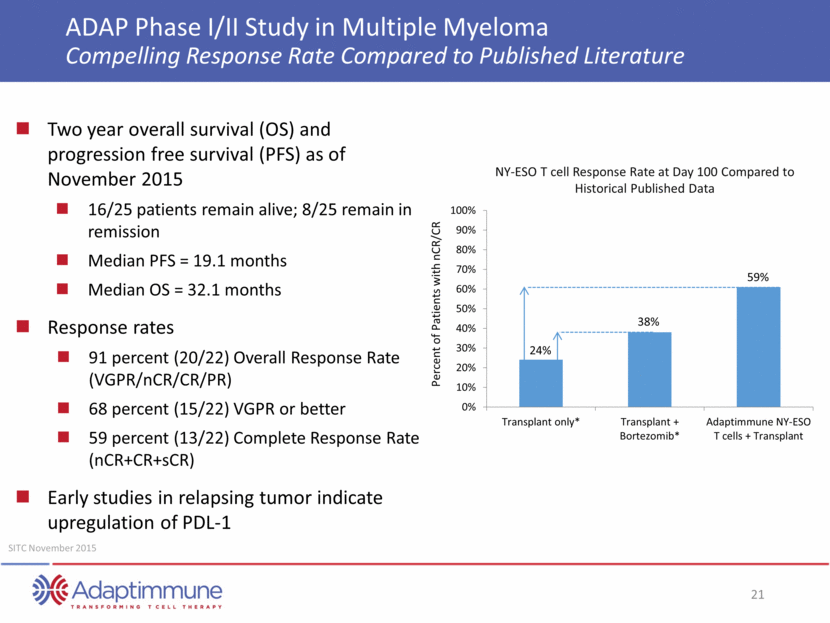
ADAP Phase I/II Study in Multiple Myeloma Compelling Response Rate Compared to Published Literature Two year overall survival (OS) and progression free survival (PFS) as of November 2015 16/25 patients remain alive; 8/25 remain in remission Median PFS = 19.1 months Median OS = 32.1 months Response rates 91 percent (20/22) Overall Response Rate (VGPR/nCR/CR/PR) 68 percent (15/22) VGPR or better 59 percent (13/22) Complete Response Rate (nCR+CR+sCR) Early studies in relapsing tumor indicate upregulation of PDL-1 SITC November 2015 Percent of Patients with nCR/CR NY-ESO T cell Response Rate at Day 100 Compared to Historical Published Data 24% 38% 59% 0% 10% 20% 30% 40% 50% 60% 70% 80% 90% 100% Transplant only* Transplant + Bortezomib* Adaptimmune NY-ESO T cells + Transplant
Recent Progress: Allogeneic program Universal Cells
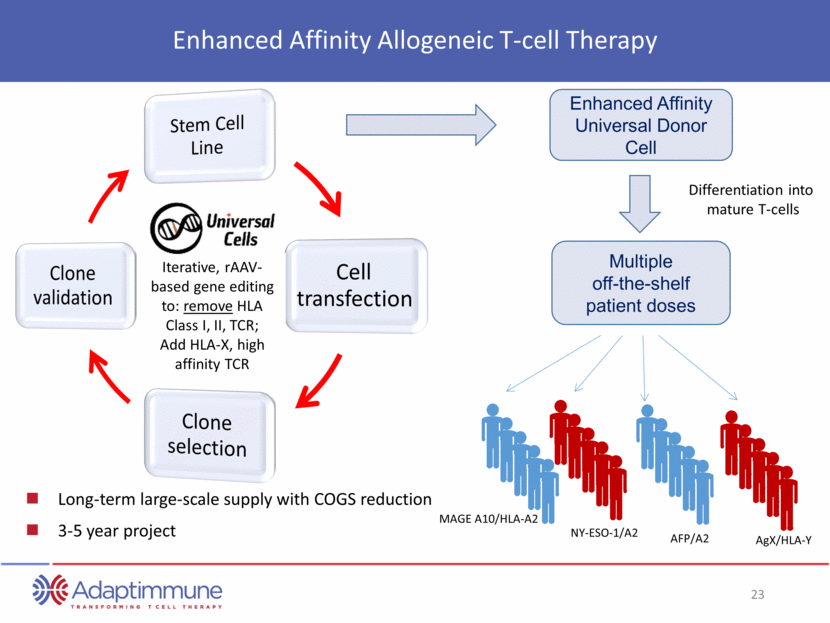
Enhanced Affinity Allogeneic T-cell Therapy Iterative, rAAV-based gene editing to: remove HLA Class I, II, TCR; Add HLA-X, high affinity TCR Long-term large-scale supply with COGS reduction 3-5 year project Differentiation into mature T-cells Enhanced Affinity Universal Donor Cell Multiple off-the-shelf patient doses MAGE A10/HLA-A2 NY-ESO-1/A2 AFP/A2 AgX/HLA-Y Stem Cell Line Cell transfection Clone selection Clone validation
Universal Cells Agreement Creating universal donor cells Exclusive Research and Collaboration Agreement for development of allogeneic T-cell therapies Potentially decreases cost of manufacture Enables large numbers of patients to be treated from the same manufacturing batch Universal Cells technology uses gene editing technology to selectively engineer cell surface proteins. No nucleases required R&D program with goal of producing a universal donor T-cell Exclusive IP license to Adaptimmune within T-cell immunotherapy field Upfront license and start-up fees of $5.5 million Development and milestone payments of up to $41 million Profit share on first product and royalty on other products
Delivery and Momentum
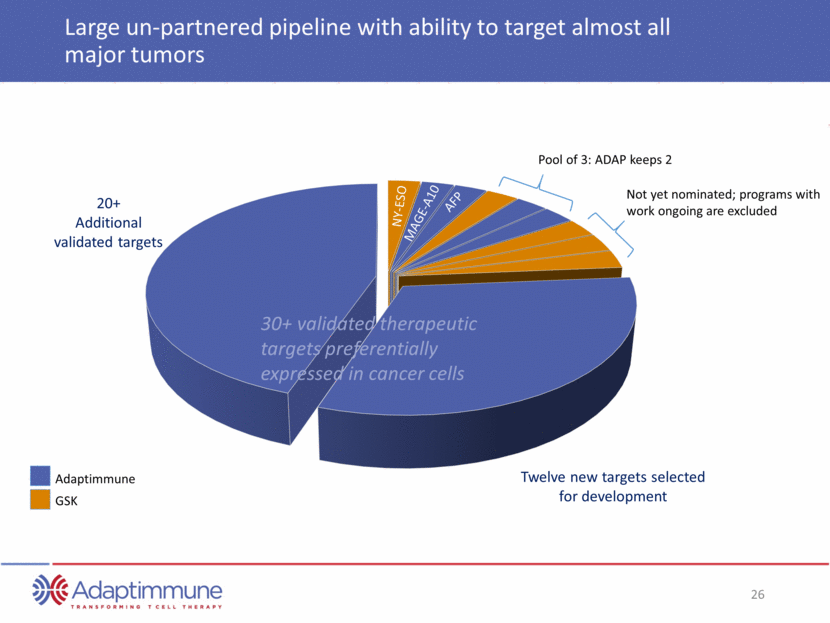
Large un-partnered pipeline with ability to target almost all major tumors Adaptimmune GSK 20+ Additional validated targets Twelve new targets selected for development Not yet nominated; programs with work ongoing are excluded Pool of 3: ADAP keeps 2 NY-ESO MAGE-A10 AFP 30+ validated therapeutic targets preferentially expressed in cancer cells
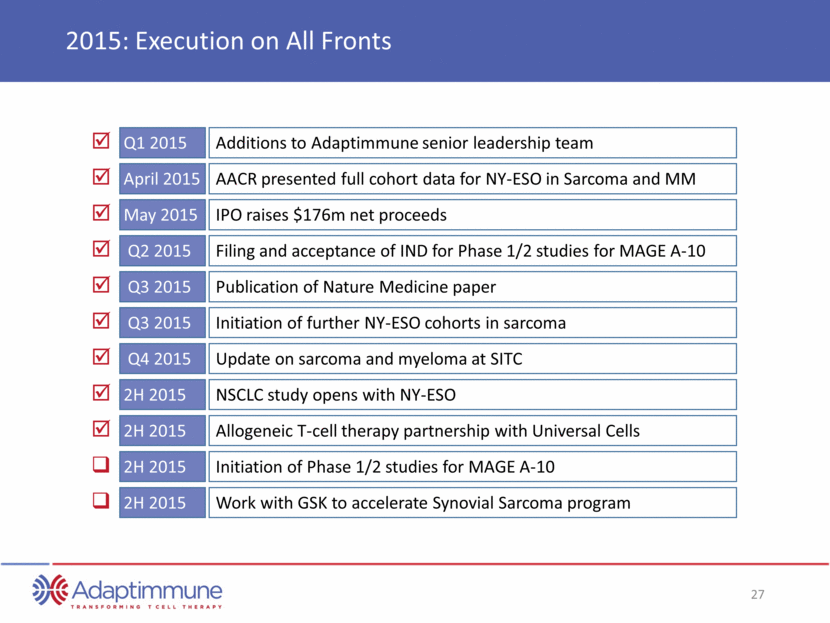
2015: Execution on All Fronts Q1 2015 Additions to Adaptimmune senior leadership team April 2015 AACR presented full cohort data for NY-ESO in Sarcoma and MM May 2015 IPO raises $176m net proceeds Q2 2015 Filing and acceptance of IND for Phase 1/2 studies for MAGE A-10 Q3 2015 Publication of Nature Medicine paper Q3 2015 Initiation of further NY-ESO cohorts in sarcoma Q4 2015 Update on sarcoma and myeloma at SITC 2H 2015 NSCLC study opens with NY-ESO 2H 2015 Allogeneic T-cell therapy partnership with Universal Cells 2H 2015 Initiation of Phase 1/2 studies for MAGE A-10 2H 2015 Work with GSK to accelerate Synovial Sarcoma program
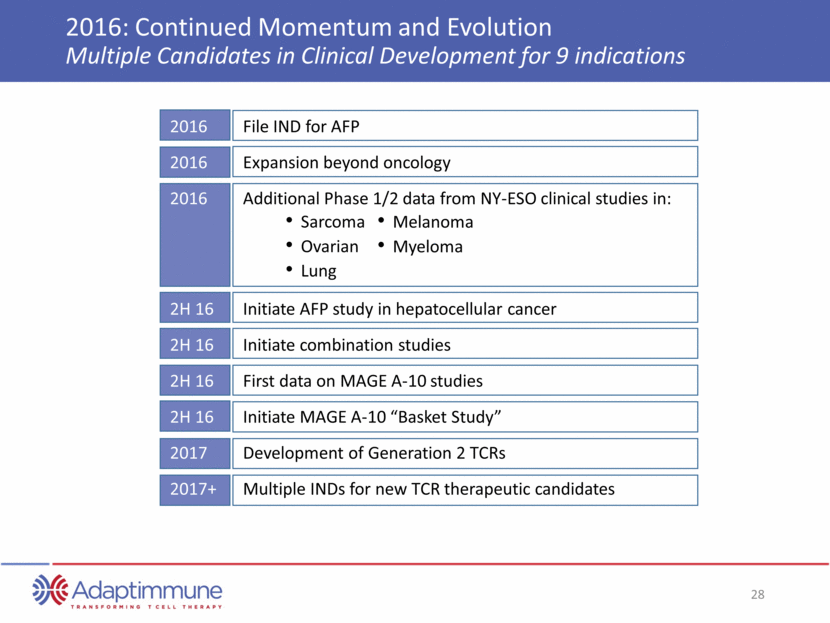
2016: Continued Momentum and Evolution Multiple Candidates in Clinical Development for 9 indications Sarcoma Ovarian Lung Melanoma Myeloma 2016 File IND for AFP 2016 Expansion beyond oncology 2016 Additional Phase 1/2 data from NY-ESO clinical studies in: 2H 16 Initiate AFP study in hepatocellular cancer 2H 16 Initiate combination studies 2H 16 First data on MAGE A-10 studies 2H 16 Initiate MAGE A-10 “Basket Study” 2017 Development of Generation 2 TCRs 2017+ Multiple INDs for new TCR therapeutic candidates
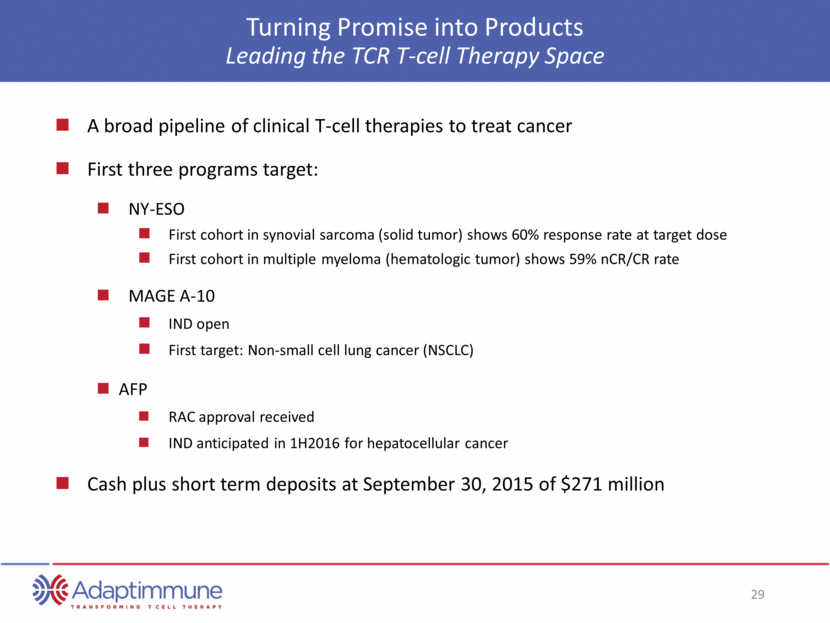
Turning Promise into Products Leading the TCR T-cell Therapy Space A broad pipeline of clinical T-cell therapies to treat cancer First three programs target: NY-ESO First cohort in synovial sarcoma (solid tumor) shows 60% response rate at target dose First cohort in multiple myeloma (hematologic tumor) shows 59% nCR/CR rate MAGE A-10 IND open First target: Non-small cell lung cancer (NSCLC) AFP RAC approval received IND anticipated in 1H2016 for hepatocellular cancer Cash plus short term deposits at September 30, 2015 of $271 million
Adaptimmune Transforming T-cell Therapy Presentation Materials December 2015
