MD Anderson Cancer Center

How our alliance with MD Anderson Cancer Center is delivering results
Adaptimmune has a multi-year strategic alliance with The University of Texas MD Anderson Cancer Center signed in 2016 designed to expedite the development of novel adoptive T-cell therapies for multiple types of cancer. This alliance utilizes expertise from clinical and translation sciences teams across the two organizations. MDACC is an important partner for Adaptimmune, both for its role as a leading US cancer center in treating patients and its translational science expertise.
Why this alliance?
MDACC is acknowledged as a key hub for the development of cell therapies - with immunotherapy being a particular focus. The collaboration between Adaptimmune and MDACC across the translational science area is accelerating our understanding of existing programs, as well as playing a key role in Adaptimmune's ongoing research and development of other new SPEAR T-cell therapies to targets in a wide range of solid tumors.
The partnership also enables access to tumor samples which assist in the validation of new targets and ultimately informs clinical trial design, while its cancer immunology core skills and expertise in performing translational medicine studies inform Adaptimmune's optimization of the efficacy and safety of SPEAR T-cell therapies.
From the 2016 press release:
Details about one of our initial projects - ADP-A2M4 (MAGE-A4) Expression
Since 2016, we have worked together on multiple projects. We reported findings for one of our initial projects at AACR 2018. Here is a link to the poster: http://aacr18.posterview.com/nosl/i/2562.

While ADP-A2M4 expression in tumor types can be estimated from published databases, it is vital to complement this with an analysis of actual tumor samples. A recent example of the excellent collaboration between Adaptimmune and MDACC is the work carried out to determine the frequency of ADP-A2M4 expression in non-small cell lung cancer (NSCLC), further informing Adaptimmune's screening strategies in this key indication.
A total of 534 resected NSCLC cases (Stage I - IV) were selected from the MD Anderson tumor repository. These samples came with associated clinical pathology information including overall survival and recurrence and were analyzed for ( ADP-A2M4 ) expression.
Expression analysis was carried out using immunohistochemistry, with the tumor samples stained and scored by MDACC scientists and pathologists. The data was analyzed by translational scientists at Adaptimmune.

Figure: NSCLC samples showing cytonuclear immunohistochemistry staining
IHC staining of tissue microarray samples, showing negative, weak, moderate and high levels of staining for ADP-A2M4.
A higher frequency of ADP-A2M4 was observed in squamous cell carcinoma (SCC) than adenocarcinoma, with 30% of SCC cases having strong ADP-A2M4 staining compared to only 2% of adenocarcinoma cases. In addition, ADP-A2M4 was seen to be expressed in both primary and recurrent NSCLC indicating that MAGE-A4 is a promising target in both of these situations.
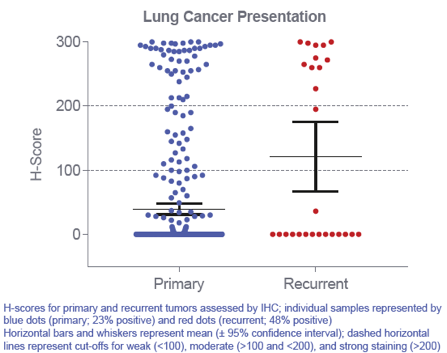
Figure: Distribution of expression across primary and recurrent NSCLC cases
A clinical trial opened in 2017 to treat patients with inoperable or metastatic NSCLC (SCC, adenosquamous, or large cell carcinoma); ovarian cancer; head and neck SCC; gastric or esophageal cancer (SCC or adenocarcinoma); urothelial tumors; and melanoma (NCT03132922). Work is ongoing in collaboration with MDACC to evaluate the expression of ADP-A2M4 in samples from the MDACC tumor repository and will be used to further improve the identification of patients who can benefit the most from ADP-A2M4 SPEAR T-cell therapy.