Preclinical autologous and allogeneic pipeline
Our deep preclinical pipeline includes both autologous and allogeneic therapies.
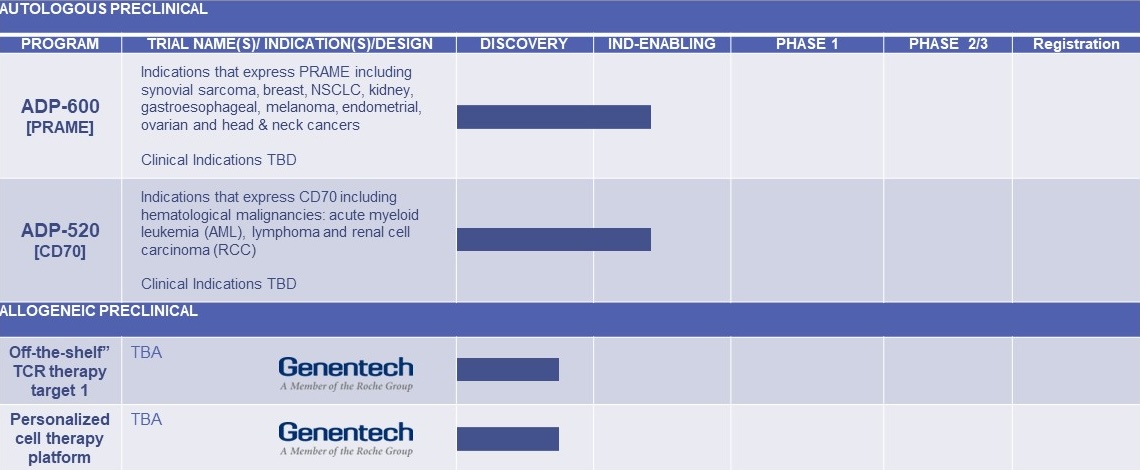
Adding to our ongoing MAGE-A4 directed autologous program, we are also focusing on the development of a T-cell therapy directed to PRAME and CD70 - both highly validated T-cell targets in solid tumors.
We have built one of the leading allogeneic T-cell platforms using human induced pluripotent stem cell lines (hIPSCs). This platform is flexible, scalable, and produces functional T-cells that kill tumor cells in vitro. We will combine this platform with everything we have learned from decades of autologous T-cell therapy research and development into a pipeline for Adaptimmune and its partners for off-the-shelf or allogeneic cell therapies. We plan to file our first allogeneic IND in 2025.
| Program [Target] | Trial Name(s) / Indications / Design | Ind-Enabling | Phase 1 | Phase 2/3 | Registration | |
|---|---|---|---|---|---|---|
| afami-cel [MAGE-A4] | SPEARHEAD-1 pivotal trial synovial sarcoma | U.S. FDA Accelerated Approval | ||||
| SPEARHEAD-3 pediatric study in tumors that express MAGE-A4 (synovial sarcoma, malignant peripheral nerve sheath tumor (MPNST), neuroblastoma or osteosarcoma) | ||||||
| lete-cel [NY-ESO] | IGNYTE-ESO synovial sarcoma and MRCLS | |||||
|
uza-cel (ADP-A2M4CD8*) [MAGE-A4] |
Collaboration with Galapagos head & neck cancer and potential future solid tumor indications | |||||
| ADP-600 [PRAME] | Indications that express PRAME including synovial sarcoma, breast, NSCLC, gastroesophageal, melanoma, endometrial, ovarian and head & neck cancers. Clinical Indications TBD | |||||
| ADP-520 [CD70] | Indications that express CD70 including hematological, malignancies, acute myeloid leukemia (AML), lymphoma and renal cell carcinoma (RCC). Clinical Indications TBD | |||||
|
--The safety and efficacy of investigational products have not been established. * Enrollment in the SURPASS family of trials with uza-cel (ADP-A2M4CD8) manufactured on the Adaptimmune manufacturing platform has been discontinued. |
||||||
Publications
Review our findings and publications.